This article appears in VICE Magazine's 2019 Profiles Issue. This edition looks to the future by zeroing in on the underrecognized writers, scientists, musicians, critics, and more that will shape our world next year. They are "the Other 2020" to watch. Click HERE to subscribe to the print edition.
There’s a chilling calculation that’s simple enough to solve on the back of an envelope: How many children’s lives will Kevin Esvelt personally be responsible for if he makes one mistake on the job?
Depending on the variables, the final figure could be between 25,000 and 2.5 million. Even the lower end of that range is horrifying. “Just imagine 25,000 dead children. It’s nearly impossible. That’s like a third of the children in Boston,” Esvelt said, gesturing out his window at the MIT Media Lab in Cambridge, Massachusetts. “Because I said the wrong thing. Because I failed to think about the consequences of my actions.”
Esvelt doesn’t need the envelope, because the calculation is continuously running in his mind: It’s a rough estimate of the number of people who could die from malaria in the next 10 years. Those same lives could potentially be saved with CRISPR-based gene drive—a technology that the evolutionary engineer conceptualized in 2013 and told the world about in 2014.
When an organism sexually reproduces, its genes have a 50/50 chance of being passed to its offspring. A CRISPR gene drive is a way to override those odds, and make it nearly certain that a trait will be inherited. It could be used to make all of a mosquito’s offspring male, for example, rendering their reproduction nearly impossible and depleting a species that kills more than half a million people per year, most of them kids. In a darker vision of the future, a gene drive could accidentally cause unforeseen ecological consequences, and turn the public against it, preventing its use.
“Nothing in my training had prepared me for that,” Esvelt recalled. Gene drive shifted his career and identity from being just a scientist in a lab to someone bound to the concern about how new technologies will challenge the ways we structure research, make policy decisions, and inform the public about choices that will affect their environments.
Esvelt has leaned in to it, making a name for himself as the morality guy. “Not since Robert Oppenheimer has a scientist worked so hard against the proliferation of his own creation,” declared a 2018 Pacific Standard profile of gene drive technology. But in the end, Esvelt’s efforts are not really about gene drive. As we await other powerful technologies to be invented—capable of fundamentally changing our environments, DNA, or health—Esvelt wants to use gene drive to set up some moral guidelines for how science and humanity decide to wield such tools, no matter what they are. Gene drive can help prepare us for whatever comes after it—the next technology that has the potential for great good, and also for great destruction.
I met Esvelt on a gray weekday morning in September, in an older part of the MIT Media Lab’s chic, glassy building in Kendall Square. The Media Lab was in the midst of a scandal regarding its director Joi Ito’s ties to the financier and convicted sex offender Jeffrey Epstein, but Esvelt made clear that he couldn’t comment on it until the investigation by MIT’s external law firm was complete. At eight thirty, the whole floor was deserted. As I waited, I wandered around the vacant cluttered desks and peered into a fish tank containing a sleeping axolotl, a cartoonish amphibian with gills that frame its face like a headdress.
Esvelt arrived a few minutes late, mentioning a delayed train, and we entered his office—surprisingly cramped for a man navigating such expansive responsibility. Plants lined the far wall, and he pulled out a huge plastic jug and dumped water on them while he opened the window. He apologized if I felt chilly, but informed me in a matter-of-fact tone that research has shown that CO2 can build up in rooms with closed doors and windows.
Lanky and pale, with a jumpy, frenetic presence, Esvelt was wearing shorts, sandals, and a blue button-down shirt that emphasized the blue of his eyes. His clothes were crisp and clean, but they looked like an afterthought, as if someone else had laid them out on a bed for him. One of Esvelt’s colleagues likened him to Doogie Howser, the fictional teenage doctor, and his face does have an exaggeratedly youthful pallor—he could pass for much younger than his 37 years.
When Esvelt was younger, he was a fellow at Harvard University’s Wyss Institute, working with the renowned geneticist George Church and a group studying CRISPR/Cas 9, a bacterial defense system that has been adapted into a gene-editing tool. Some bacteria store pieces of virus DNA from previous attacks and then use an enzyme, Cas 9, to chop up any DNA they encounter in the future that matches those fragments.
Scientists realized that they could manipulate this system to cut any DNA sequence they wanted, by changing the DNA that CRISPR/Cas 9 used as its guide. As a gene editing technology, CRISPR single-handedly revolutionized the field by offering a way to precisely cut DNA. But Esvelt was bored. He’d been working on CRISPR for around nine months, and that was long enough for him to predict the majority of what its future applications would be. Or so he thought.
On a spring day in 2013, Esvelt passed through Emerald Necklace, a park on the way from Boston's subway to the Longwood Medical area. As he walked through the greenery, he thought about how evolution would overpower whatever CRISPR edits a scientist made to an animal’s DNA.
Organisms that sexually reproduce get two copies of each gene, one from each parent. Let’s say a scientist edited a mosquito: When that genetically modified mosquito mated with an unmodified one, only one copy of the edited gene would get passed down. This would dilute the genetic modification after just a few generations, especially if the edit wasn’t benefiting the mosquito’s survival.
But what if you could use CRISPR to edit a mosquito to have a new gene and carry the tools to alter that gene in its offspring, too? When an edited mosquito mated with an unedited one, the offspring would inherit a CRISPR system that would chop away the second, unedited version of the gene.
When there’s DNA damage to one gene, a cell tries to repair it, using the other copy as a template. In this case, the other copy would be modified, and the mosquito would be left with two copies of the edited gene, guaranteed to pass it along to its offspring. The change would sweep through a population without human intervention after only a few edited mosquitoes were released into nature.
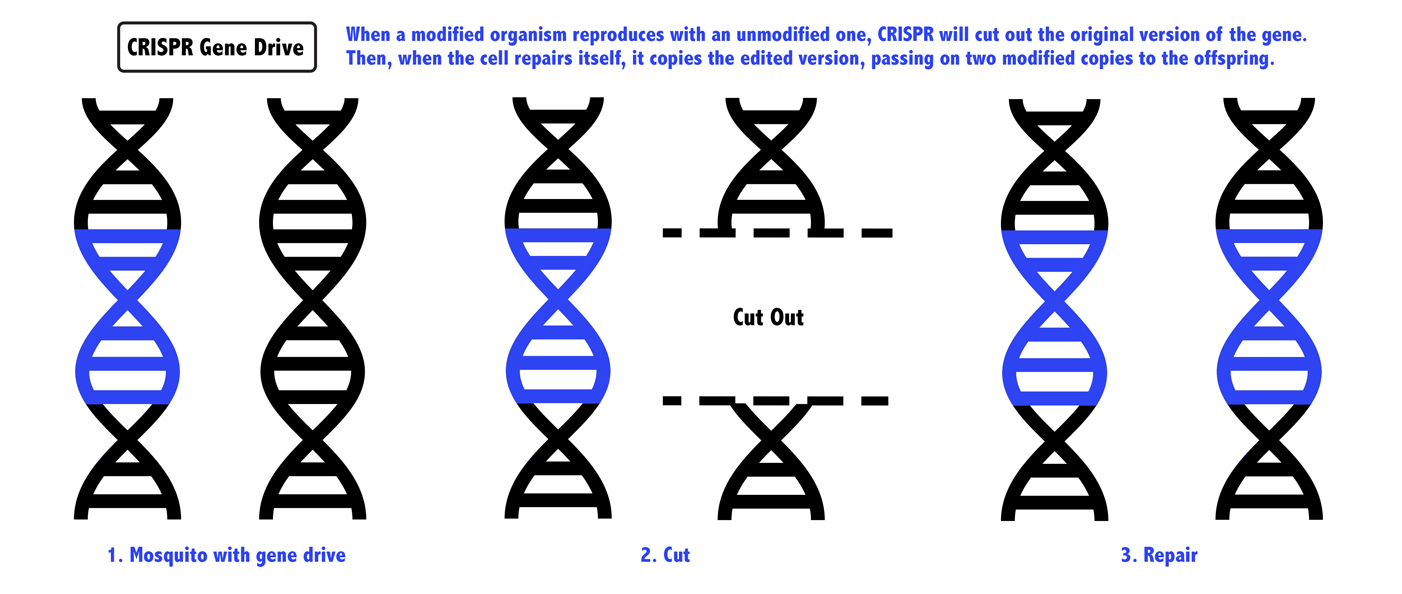
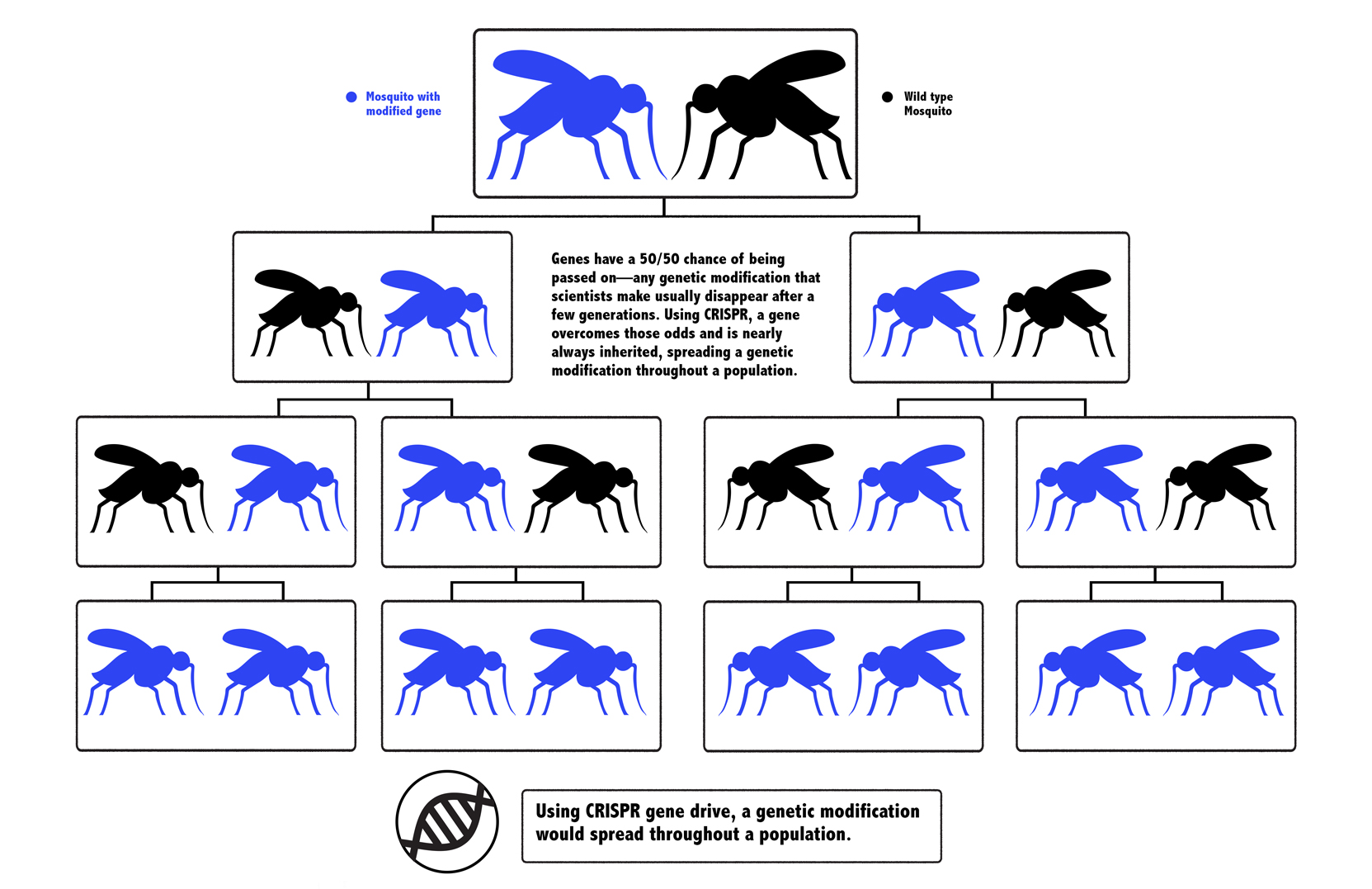
The idea was a riff on something called a gene drive, which occurs naturally in biology: It's when a gene proliferates throughout a species, even if it's not necessarily contributing to the survival of the organism. Since the 1940s, scientists have tried to harness gene drives for public health. In 2003, the biologist Austin Burt proposed that a gene drive could be used to stop malaria, but actually creating a gene drive was expensive and difficult. Esvelt’s insight of using CRISPR to facilitate gene drive was the missing ingredient to make it not only feasible, but relatively easy.
Esvelt was elated. Burt was right—a gene drive could, theoretically, be used to eradicate malaria, but that was only the beginning of what a CRISPR gene drive could do. It could address all the other mosquito-borne illnesses and other diseases that jump from animal populations into people. "If we edited all the wild birds to be immune to influenza, then you wouldn’t have avian flu jumping into humans and pigs and jumping into us," Esvelt said. "We could edit all of the parasitic worms that cause so much suffering.”
After a full day of marveling at the possibilities, this honeymoon period came to a sudden halt. It wasn’t that “we” could do anything—one single scientist could do it. Esvelt could do it on his own. CRISPR was a simple tool, and he had access to it at his own lab, as did many others.
“I thought, Whoa, whoa, whoa,” Esvelt said. “It’s one thing for humanity to be able to do this. It’s quite another for individual researchers to have this kind of power.” He started to spiral: How dangerous was his idea—what ripple effects on an ecosystem could changing the DNA of one species have? Would it be uncontrollable—since CRISPR gene drives would be self-propagating, could it be stopped if something went wrong? Could it be blocked? How fast would it be? Could it be detectable—or could someone release a gene drive in secret? Esvelt ruminated for a couple of weeks, then he went to George Church, and asked for advice.
Church told me that when he first hired Esvelt, he didn’t see him as particularly interested in safety, ethics, or policy. “It was only when they started talking about gene drives that he started to get antsy,” Church said. “And everything followed from that point on.”
“It’s one thing for humanity to be able to do this. It’s quite another for individual researchers to have this kind of power.”
When Esvelt finally did publish his gene drive paper with several of his coworkers in eLife in 2014, they intentionally hadn’t executed it yet. They wanted to introduce the concept, consult with experts in various disciplines, and invite criticism; a majority of the paper is about safety, control, and ethical concerns. When they did make a drive for the first time in 2015, in yeast, they simultaneously built a second drive that overwrote the first and restored it. “Reversibility was one of the first things that we worried about,” Church said.
In the spirit of harnessing CRISPR gene drive’s power, Esvelt and his colleagues have since developed another approach, dubbed a daisy drive. It is a gene drive designed to fizzle out. It won’t self-propagate forever, but uses a series of connected drives that eventually do succumb to natural selection. Esvelt has also been brainstorming ways to keep a drive from spreading by making it so that when an edited organism procreates with a non-edited one, it will create half as many offspring—a kind of genetic electric-dog fence.
Despite these efforts, the public at large remains wary of the technology. Media organizations regularly use the word “risky” to describe it. In 2016, a group of 30 environmentalists and scientists called for a moratorium on gene drive, asserting that any release would be too dangerous. In 2018, a similar proposal at the 2018 UN Convention on Biological Diversity was rebuffed in part by an open letter from more than a hundred scientists, including Esvelt, Church, and Burt, arguing against a moratorium.
Esvelt doesn’t wholly disagree. He’s often a source in articles about the risks. Since the 2014 paper, he’s spoken publicly about how stringent the standards for using original self-propagating gene drive should be, and the need for any trial to start small and scale up. (Esvelt does think that malaria is one of the very few cases where the full, self-propagating drive’s benefits can rival the risks.) Still, he exists on his moral seesaw: What are the consequences of doing nothing? Of being too cautious, spreading too much hesitation about gene drives? How many people could they be saving?
In a way, Esvelt got lucky with gene drive. As far as big, dangerous ideas go, it’s better to have one that spreads through reproduction—it’s slow and limited to organisms that sexually reproduce (not bacteria and viruses). You can’t hide it. Sequencing DNA would quickly reveal that a CRISPR gene drive is present.
But the dangerous idea that Esvelt walked away with was not gene drive. It was that one day a technology could not exist, and then the next day, it could. Its sudden genesis could threaten to affect the lives of millions of people who did not consent to it, propagate on its own, and have completely unpredictable side effects. At any moment, we could be on the cusp of the next big dangerous idea in artificial intelligence, or human-machine interfaces, or synthetic biology. “It doesn't give us any time to think about the implications, possible offenses, and so forth,” Esvelt said. “Everything is on one person. And so that was a deeply uncomfortable position to be in.”
Esvelt grew up between Portland and Seattle, where it’s not hard to be stupefied by nature’s grandeur—“Lots of running around in the forest.” His mother was an elementary school teacher and his father worked for Bonneville Power Administration, but they recognized that their son was entranced with biology, especially dinosaurs. Though he was only in third grade at the time, he read Jurassic Park when it first came out, and thought he would grow up to be a paleontologist.
In the sixth grade, his parents took him to the Galápagos Islands. The trip led to Esvelt reading the writings of Charles Darwin, whom he still speaks of with reverence. Evolution gave Esvelt a guide to how life works—“nothing makes sense in biology except in the light of evolution,” as the geneticist Theodosius Dobzhansky famously wrote. But though evolution is comprehensive, it is far from a rule book.
Esvelt called evolution a “blind idiot alien god” (a nod to H.P. Lovecraft), and it was a difficult realization for him that his guidebook to life had no moral compass. “It evolved things that cause horrific suffering, and it doesn’t care because there’s nothing there to care,” he said. “It’s just an emergent process. But we care.”
Esvelt cared a lot—he described himself as a radical environmentalist in high school, a teenager who loved the natural world, and was hell-bent on stopping humans from destroying it. Ben Brown, a professor in astrophysics at the University of Colorado Boulder, who went to high school and college with Esvelt, said that they would often discuss problems on the bus to track and field competitions—like humanity’s impacts on global ecosystems and how to support growing populations without running out of natural resources.
Esvelt’s environmentalism was different from the radical “tree-hugger, chainsaw-sabotaging, bike-riding-for-peace-and-world-saving brand of environmentalism,” that was common in the Portland area, Brown said. Esvelt was more about radical ideas. He once felt that since the sun would eventually destroy Earth, the only way to preserve life long-term would be directed panspermia, or shooting out some version of life into the universe to develop elsewhere. (Esvelt now thinks directed panspermia is morally reprehensible.)
In college, Esvelt continued to generate big, radical ideas, Brown said. His undergraduate thesis was on studying fertilin beta, a sperm surface protein. His goal: to figure out a way to induce reversible male sterility, so that every act of procreation should be intentional; he thought that making a baby should be an opt-in rather than opt-out situation (and that the responsibility should fall to men for a change).
Brown remembered talking with him about it, saying, “Kevin, on the one hand, this makes perfect sense. That’s a great idea. But how in the hell could you ever give corporations control over the fertility of mankind, of humankind? How is this ever going to work? College-age Kevin had a hard time seeing the dark sides that could come out of those choices and technologies, and largely just saw the benefits and advantages that could come out of them, and ways to try and solve problems that he saw as critical to solve in the world.”
That all changed with CRISPR gene drive. The last time Brown saw Esvelt in Boston, he reflected on just how much time Esvelt dedicated to the potential outcomes of his work. “He had spent as much, or more, time thinking about some of the long-term moral and ethical impacts as most of my colleagues would spend on just the straight-up research parts."
Esvelt’s lab today is called Sculpting Evolution. On paper, their mission is to research evolutionary and ecological engineering. But Esvelt has greater ideological and philosophical stakes. “Our long-term mission is cultivating wisdom,” he explained. He sees his work as not only to sculpt the evolution of living systems, but to help shape the evolution of technology, and provide some of the morality that evolution lacks. The young man with the big ideas started to grasp just how big they were. “The future of our civilization is primarily going to be determined by the technologies that we invent and the wisdom with which we choose whether, when, and how to use them,” he said.
One place Esvelt is trying to exercise this wisdom, and what careful implementation of a new technology looks like, is on Martha’s Vineyard and Nantucket in Massachusetts. The project is called Mice Against Ticks. In the summer of 2016, Esvelt’s lab reached out to the Nantucket Board of Health to present the possibility of using a gene drive on white-footed mice in the area that infect ticks with Lyme disease. It was an intriguing pitch, said the director of the health and human services department, Roberto SantaMaria, because about 40 percent of the island’s population has had Lyme disease at one point.
Esvelt and his PhD student Joanna Buchthal gave a presentation explaining gene drive and CRISPR to the entire group. On a Facebook group for yearlong residents, the page lit up with discussions about Esvelt and his proposals. The reactions, in SantaMaria’s recollection, were mixed: “Everyone’s mind jumped to Jurassic Park.”
The residents viewed Esvelt as an “egghead” swooping in from Boston to alter their land, SantaMaria continued. But Esvelt kept coming back—in the first year, Esvelt and Buchthal gave a handful of presentations. Every few months, they talked to 30 or 40 more people, and even more watching a livestream online. “The openness of his research, the openness and his willingness to prevent anything from going wrong really caught us off guard,” SantaMaria said. “It really made us trust him.” Sam Telford, an epidemiologist who has been focused on Lyme disease and other tick-transmitted infections for 35 years, said that Esvelt has given so many presentations, “it rivals my speaking on both islands over, I don’t know, the past 10 or 15 years.”
But despite all that time and energy, Esvelt is currently not pursuing a CRISPR gene drive—the communities said they preferred him not to. “One of the things they said was to try to keep it as natural as you can,” Esvelt explained. Instead, they are using a genetic modification to make mice that continuously produce the antibodies for Lyme disease, and to make offspring able to inherit that immunity from their parents.
One could argue that it is also not “natural” for a mouse to inherit resistance from their parents—that’s not how immune systems “naturally” work. Esvelt knows the categorization is subjective, but he is putting his money where his mouth is. “If it’s your environment, it’s your call,” he said. “We don’t live there. We’re not going to be affected if you decided to do this.”
Telford admitted that the project would likely be a lot easier using a gene drive, but that’s not the point. “The history of public health is that there are many examples of interventions that are forced down people’s throats,” Telford said. “[Kevin] has a very refreshing approach of actually involving the communities from the very start, letting them know, ‘Look...This is what we want to do. Before there’ll be any kind of release in your communities, you will vote and you will have an opportunity to say no.’”
Esvelt sees his role as either a facilitator or enabler, not someone making a pitch. He’s not about finding a place to fast-track a trial for gene drive, but to develop a framework for how to approach communities with genetic technology in the future—in a way that’s inclusive, respectful, and starts small. To cultivate the most wisdom, we need to encourage collective power without individual access—not create situations where a single person has access to the proverbial big red button, he said. Even (or especially) if that person is him.
“Mice Against Ticks in and of itself does not exist because I think Lyme disease is the most important problem in the world that I could be working on,” Esvelt said. “Rather, it exists because I want to change science and make it more open in this area of ecological editing. The best way of doing that is choosing a local problem with particularly relevant communities who could set an example for how things should be done elsewhere in the United States, and ideally elsewhere in the world.”
As technology advances and trickles out into the real world, science will continue to face this hard truth: It has to grapple with people’s values and moral beliefs as much as the hard data and facts. “We base our discussions on scientific evidence as well, but it’s not the only factor that comes into play,” said Jennifer Kuzma, a professor and the co-director of the genetic engineering and society center at North Carolina State, who has been studying the governance of emerging biotechnologies for more than 20 years. “It’s informed by science but not dictated by science.”
Esvelt thinks scientists come with their own values and biases too. “People likely to become scientists do so because they have a particular set of predilections and traits that encouraged them to think about things in a reductionist, materialist manner,” Esvelt said. “It’s a self-sorting group. It doesn’t mean that you should have that much power because of your differences. It does not mean that you are unusually wise.”
Scientists are asked to specialize in niche subjects, “down, down, down, until you’re in the lightless caverns that have never been explored,” Esvelt said. He finds this dangerous. Communication within the sciences is just as important as with the public; with highly specialized people working in isolated fields, they could accidentally unleash new technologies.
"The future of our civilization is primarily going to be determined by the technologies that we invent and the wisdom with which we choose whether, when, and how to use them."
In 2015, at the University of California in San Diego, researchers were studying genes that create vein patterns in fruit fly wings. They figured out they could use CRISPR to make different mutant flies, and also make certain flies yellow, to tell them apart. It was a CRISPR-gene drive. They also recognized the changes could be inherited and self-propagate, and took safety and containment measures. But Esvelt was still concerned: If one of those flies had gotten out, it could have made a whole bunch of local fruit flies yellow. Not necessarily dangerous on its own, but what if a simple accident like this sparked a media frenzy, and turned the public against gene drive, halting ongoing malaria research?
One accident could affect the whole future of this work. In 1999, a patient died while participating in a gene therapy trial at the University of Pennsylvania, which "really slowed down...the field for a decade at least," according to Kuzma. "Could it happen in the case of gene drive? Yeah," she said.
According to Esvelt, the way science is structured now, we can only expect more mistakes. Researchers work essentially in secret and don’t reveal what they’re working on until they’re done. “That’s not fair,” he said. “But at the same time, these consequences are very real.”
Radical transparency isn’t realistic for everyone—in a world of competing for funding and publication in top journals to secure said funding, laying all your cards out on the table could leave PhD students without pay or leave smaller labs vulnerable to others stealing their ideas.
Telford said that Esvelt has the “luxury of being able to think of these bigger pictures that we scientists in general really should have in the back of our minds, because he’s in the Media Lab at MIT. I was kidding him a while ago, saying, ‘Well look, you’re at MIT, this is like a top-echelon science institution in the world. Shouldn’t you be writing real hard science papers?’ And he has this big smile on his face and says, ‘No, no, I’m in the Media Lab. The only thing that matters to me is making an impact socially and culturally and politically.’”
It’s one of the reasons Esvelt pushes so hard on communication and openness, because he has the privilege of being able to do so. But he hopes that even a “typical” scientist will start to realize that they don’t operate in a vacuum. Research choices can greatly impact people far removed from the lab bench, just like decisions about who to accept funding from can have unexpected and sinister ripple effects.
Do scientists regularly calculate the consequences for their actions? Have they added up the number of lives, or the amount of suffering, they could cause or absolve? “The answer is no, they haven’t,” Esvelt said. “You asked what frustrates me about the world. That’s what frustrates me, that people don’t think that way. They don’t connect morality to mathematics.”
As we spoke, I started to realize that while Esvelt is applauded for exhaustively involving the communities he plans to work with, lauded as an advocate for openness and cooperation—all true—there is also a piece of this tireless cooperation that is just as much for his sake as it is for theirs; to disperse that responsibility in some small way, and to ask for help, because he holds himself personally responsible for the consequences of whatever may go wrong.
"I’m morally responsible for all of the consequences of this technology," he said. "Even if someone else uses it and screws it up, that’s still, to some extent, my fault.”
Esvelt has been described by other journalists as haunted, and I can see how people might think that. But while he seems to fully sense the gravity of his position, he is also an upbeat, excitable person. “I like drama more than is good for me, perhaps,” he said. “But on the whole, I’m a pretty cheerful person.”
His worldview similarly contains a number of contradictions. He believes that we need technological advances to continue our “ascent up the tree of knowledge,” but he also thinks that that same tree could bear fruit that threatens the stability and safety of human life. He insists we need to pay attention to people’s values and take them seriously, yet also believes those same values could be completely wrong. “The moral worldviews of humans have changed tremendously over the past few centuries,” he said.
So how do we decide what to do? It’s an age-old philosophical question, and one Esvelt is intent on bringing to the sciences. And I got the sense from him that while he takes his mission very seriously, he also enjoys it. When debating moral reasoning, his already quick-paced speaking speeds up, invigorated by the discussion. What is the moral code that one should follow? Should we focus solely on the consequences of our actions to decide? Or on the integrity of our intentions?
Do scientists regularly calculate the consequences for their actions? Have they added up the number of lives, or the amount of suffering, they could cause or absolve? “You asked what frustrates me about the world. That’s what frustrates me, that people don’t think that way. They don’t connect morality to mathematics.”
He told me that he isn’t a fan of using consequentialism on a day-to-day basis, and it relieved me to know that Esvelt doesn’t apply his mortality calculations to everything. He doesn’t, for example, stand in the produce section of the grocery store counting up deaths when comparing organic to non-organic bananas. In everyday life, he turns to other philosophical role models, like Aristotle, and the Stoics Marcus Aurelius and Seneca. Aristotle’s view on ethics was that morality should be practical and not just relegated to theory. If a person wanted to be virtuous, they must do virtuous things, and not spend their time studying and pontificating about what virtue is. Esvelt said that in his life, he tries to combine that with a crucial insight from the Stoics, which is: If there’s nothing you can do about it, don’t stress.
The thing is, though, the range of things that Esvelt can affect with his actions is a bit larger than, say, mine. I cannot personally change the DNA of a malaria-carrying mosquito, or a mouse carrying Lyme disease. Esvelt can. Can philosophical wisdom help him with the weight of that burden? Would Esvelt be happier if he didn’t need his envelope calculations, if he hadn’t come up with CRISPR gene drive, if he didn’t have to watch his words carefully, or think about the moral ramifications of every action?
“I wouldn’t trade my job for the world,” he said, smiling. After a pause he continued, “I mean, I would. I would totally trade my job for the world. I would trade my life for the world. That’s sort of part of what comes with this, right?”

from VICE https://ift.tt/2XobJf6
via cheap web hosting
No comments:
Post a Comment