Getting tested for COVID-19, the disease caused by the new coronavirus, has been difficult and confusing. Mistakes were made by the CDC, FDA, and the federal government that delayed the roll-out and accessibility of the tests (unless you're a celebrity). South Korea, one of the countries that has best contained their outbreak, tested 40 times more people per capita than the United States, a contributing factor to their success.
Yet even as some states finally start ramping up their testing of sick people, researchers are calling out the importance of a second kind of test that could provide critical information about the pandemic: one that could identify the virus’ antibodies in people who have recovered from their symptoms.
The tests that are currently available are for people who are sick right now. They look for coronavirus genetic material in nasal swabs to confirm a person has COVID-19, and not another infection.
For those who have already recovered, this test won't work, since the virus will have mostly left their system. A serological test, on the other hand, could tell if a person already had COVID-19 by detecting antibodies that the person's body produced to fight the virus.
Last week there was good news on this front: A group of international researchers announced they had developed such a test on the preprint server medRxiv (meaning it is not yet peer-reviewed).
Some private companies overseas have started to develop and sell similar tests, but the preprint is significant because it shows exactly how the researchers did it, and they used equipment many labs already have. It paves the way for others to set up and execute the test themselves. One of the preprint's authors, virologist Florian Frammer at the Icahn School of Medicine at Mount Sinai, will be shipping out necessary ingredients for other labs to do their own testing, and start processing samples this week. The FDA relaxed its rules on body-fluid tests, Reuters reported, saying these tests can go to market without full agency review and approval.
To make the test, the researchers made their own version of the spike on the surface of the virus along with one piece of the spike called the receptor binding domain. (They made their own so they wouldn't have to work with a live version of the virus.) These bits of the virus are the ones the body would make antibodies to since both are key in how the virus attaches to and takes over a human cell.
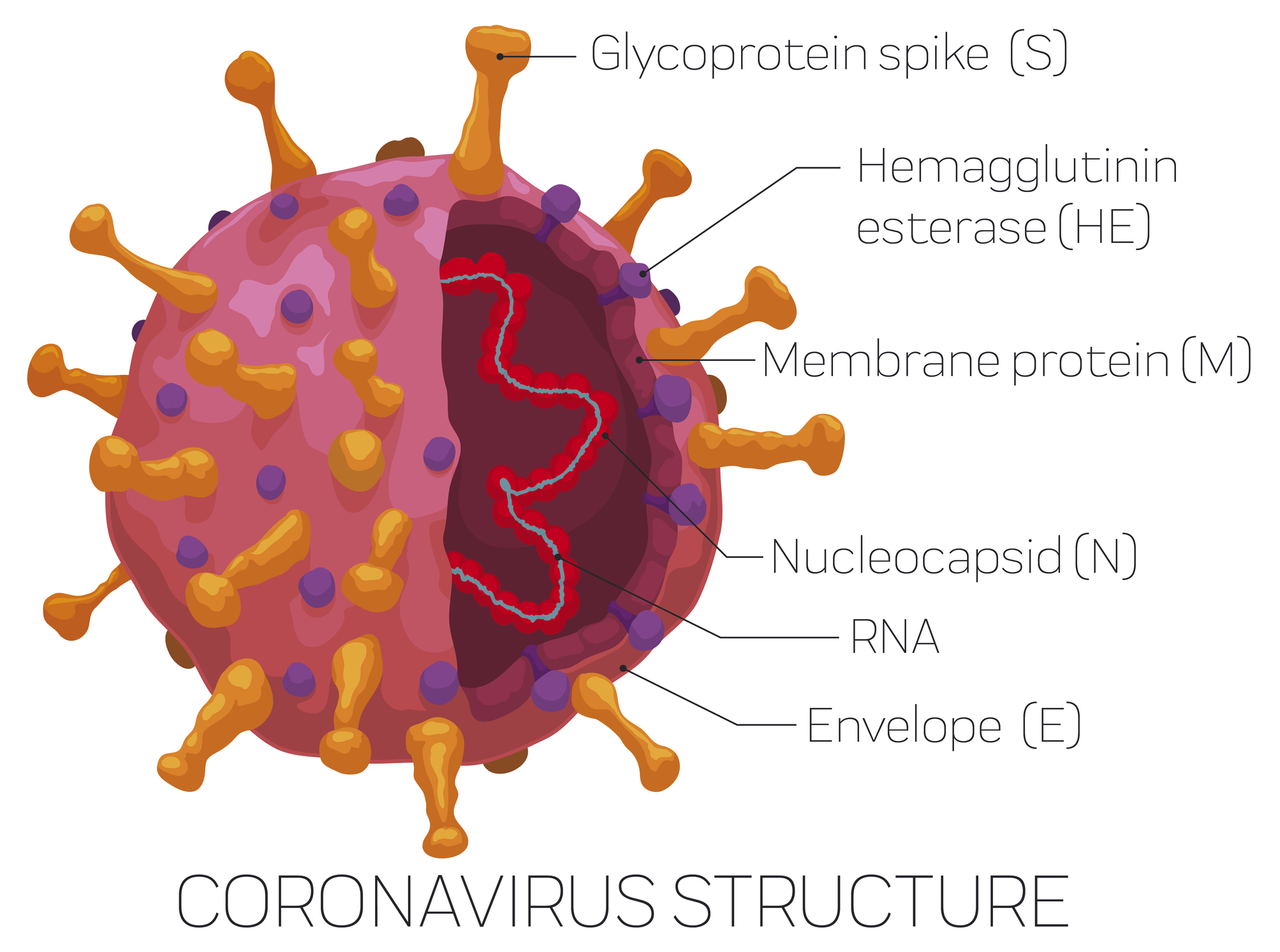
Then, they used a test called an enzyme-linked immunosorbent assay, or ELISA, which has been frequently used to detect other antibodies. Essentially, the researchers put their homemade viral bits onto a plastic plate and then spread a person’s blood sample over it. If the blood contains antibodies that pair up with the new coronavirus proteins, the ELISA plate changes colors.
In more than 50 samples from people who never had COVID-19, and three people who had been exposed to it, the researchers found that only those who had been infected by SARS-CoV-2 set off the test—indicating it could be a valuable new tool in our arsenal during this pandemic.
This kind of antibody testing, if widespread, could tell us how many total people have been infected, what the true fatality rate is, and could be crucial in deciding which healthcare workers should interact with highly contagious patients. (This is based on evidence that people who already contracted and recovered from the virus develop immunity to the virus for a couple months at least.)
The blood of people who produced a lot of antibodies might even be a treatment option for those with COVID-19, called convalescent plasma. Groups of scientists are now expressing support for that idea, and want serum to be given to patients with compassionate-use exemption from the FDA. (Plasma has been used as a treatment for polio, measles, and mumps before we had vaccines for those diseases, along with SARS and Ebola.) Governor Andrew Cuomo announced that it will be tried in New York starting this week, in which doctors can get case-by-case permission from the FDA to give their patients plasma.
To do any of these things, though, testing people's blood for antibodies needs to come first. I spoke to the preprint's first author, Fatima Amanat, a PhD candidate in Krammer's lab, about the test and how it works.
VICE: What happens when somebody is infected with the virus and they recover?Fatima Amanat: Once you get exposed to a foreign substance—it could be a bacteria or virus—your body has an immune response against it, and starts making antibodies. There's different classes of antibodies. Antibodies such as IGMs come up very quickly after infection on day three or day four. There are antibodies that are long-lived that come a little later on around day six or day seven that are called IgG antibodies. These antibodies provide us long-lived immunity.
Once you have really good long-lived antibodies in your blood, some of these antibodies can be very good at neutralizing the virus. When I say neutralizing the virus, I mean that these antibodies can bind to the protein on the surface of the virus and block it from entering cells. It basically blocks the spread of the virus.
If a person had COVID-19 a month ago and you tested them now, would those antibodies still be there?
For sure. There's a special class of cells called memory B cells—we call them that because it's like they have memory. Once your body sees that same foreign substance such as the virus, again, those cells remember, immediately get activated, and start producing more antibodies again. If I took your blood and you were infected last month, I could probably detect those antibodies on most serological tests.
How does your test pick up antibodies for the new coronavirus only, and not antibodies for other viruses or bacteria?
The virus has this one protein on the surface that it uses to enter human cells called the spike protein. What we’ve been doing is we took an ELISA test we had in the lab, and coated it with the spike protein. Then, if your blood has antibodies against this specific protein, our test can detect it. Antibodies are highly, highly specific. They don’t just bind to any normal target. An antibody that’s specific to SARS-CoV2 will only bind to that, and not anything else.
In the preprint, we tested blood from a person with another human coronavirus, NL63. We saw that that patient’s serum doesn’t react to the SARS-CoV-2 at all. It shows that the test is very specific and will not tell me, “Oh you have SARS-CoV-2,” when you actually don’t.
Wow—so it doesn’t even respond to other coronaviruses.
Exactly. NL63 is a coronavirus also and we wanted to use that sample to illustrate that we are not getting specific or random binding to our target protein.
A lot of people who were sick but didn't have access to a test want to know if they had it or not. Is that a service your test could provide right now?
There are companies out there making a rapid [serological antibody] test that you could probably buy. It’s being manufactured in South Korea or China. I think people who are curious and would want peace of mind could do something like that. But the main purpose for our test was to identify people who are doctors or nurses who've already had COVID-19 and have recovered from it, because those could be our front-line responders.
Right now, if you have a highly infectious COVID-19 patient, it's really a problem to send a nurse who is not immune to it already because she’s so susceptible. Our test would be for all the doctors and healthcare providers, to see if they're already immune to it and have antibodies.
What could we learn about the virus once we have more wide-scale antibody testing available?
One big problem about the virus is that a lot of cases are mild or asymptomatic. You could be walking down the street and have it and not know. We could understand through a larger [antibody testing] study what the total infection rate of the virus is.
Re-infection is also a big question in the field right now. And unfortunately, we just don't know yet [if you can be re-infected after having it]. We're also not sure if this will be a virus that will just disappear and not resurface, or it could become a seasonal virus like the influenza virus. If it resurfaces, we could identify those same people that we tested first and re-test them to see how their immune response is [several months later].
How quickly can these antibody tests be disseminated?
Every lab is pretty equipped to do it, but the only unique thing you need is the spike protein of the SARS-CoV2, and the RBD, the receptor binding domain. We’ve been actually sending and shipping these out to other labs and medical centers in the country and the world who are asking us for it. They say they're going to set up the same tests there. I think it will be a matter of a few weeks when this will be very widespread.
That said, I think there is a problem with capacity. Unfortunately right now our focus is to work with the doctors and the patients and nurses. I think in the future, I would definitely want to [take the test] myself personally. But I think at the moment when there's such limited capacity and such limited staff, it's hard to do with the general population.
We need to keep working on it. It's really an important test right now. We only can really see the total infection rate of the virus with this test and we can detect people who are asymptomatic and didn't get tested.
How does this kind of antibody test play a role in developing new treatment options?
People are looking at serum for therapeutic use. In the Ebola epidemic a few years ago, people were treated using serum from people who had already recovered from Ebola and giving it to another patient as a treatment. So this type of test is great to identify donors all for convalescent plasma therapy.
That would be like me, if I already had it, giving my blood to somebody else?
Exactly. This type of test could identify donors for coalescence serum therapy. If I take your blood and I'm like, "Oh, you already had a lot of antibodies on the test," you could be enrolled and be a donor and you could save another person's life by donating your blood.
The preprint also found that, based on the immune response from the people without COVID, it seemed like we have no existing natural immunity to this virus. How could you tell that and what does that mean?
The rest of our subjects were regular donors who hadn’t had COVID-19. Some of them had other diseases, like that one person with NL63. We could see that these samples are not reacting at all to the SARS-CoV-2 proteins. Basically when you have such a big number of samples that are not reacting at all, you're showing that there is no preexisting immunity in the human population at all.
It means this is a novel virus that has never surfaced in the human population before. People have no immune response to it prior to this. This differs from something like influenza which has been circulating seasonally every year so your body has immune response already. Every time you get exposed to it you just get a boost of an immune response. We're totally naive to this virus, and it contributes to how widely it has spread.
Sign up for our newsletter to get the best of VICE delivered to your inbox daily.
Follow Shayla Love on Twitter .
from VICE https://ift.tt/2WGjuyN
via cheap web hosting
No comments:
Post a Comment